What Is A Calorimeter And Why Would It Contain Water . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. For example, when an exothermic reaction. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the.
from www.tec-science.com
a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the.
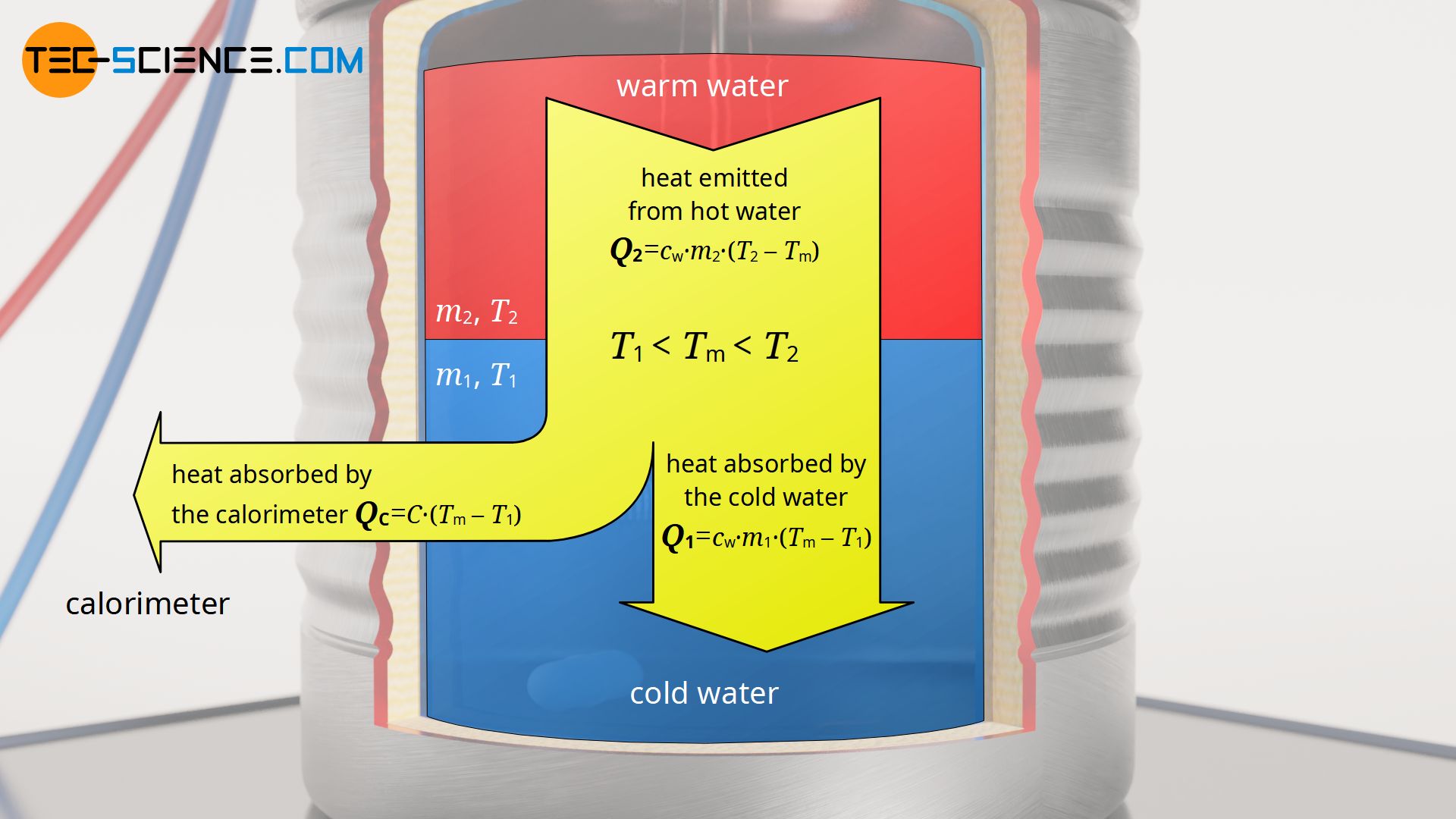
Calorimeter to determine the specific heat capacities of liquids tec
What Is A Calorimeter And Why Would It Contain Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From cider.uoregon.edu
Calorimetry Computer Simulation HTML5 CIDER What Is A Calorimeter And Why Would It Contain Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. thermal energy itself cannot be measured easily, but the temperature change caused by. What Is A Calorimeter And Why Would It Contain Water.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, What Is A Calorimeter And Why Would It Contain Water calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be.. What Is A Calorimeter And Why Would It Contain Water.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses What Is A Calorimeter And Why Would It Contain Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. What Is A Calorimeter And Why Would It Contain Water.
From manueldesnhray.blogspot.com
Identify What a Coffee Cup Calorimeter Measures. What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. . What Is A Calorimeter And Why Would It Contain Water.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter. What Is A Calorimeter And Why Would It Contain Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so,. What Is A Calorimeter And Why Would It Contain Water.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is A Calorimeter And Why Would It Contain Water For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. a calorimeter is a device used to measure the amount of heat. What Is A Calorimeter And Why Would It Contain Water.
From www.animalia-life.club
Calorimeter Diagram What Is A Calorimeter And Why Would It Contain Water thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. a calorimeter is a device used to measure the amount of heat. What Is A Calorimeter And Why Would It Contain Water.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so,. What Is A Calorimeter And Why Would It Contain Water.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical,. What Is A Calorimeter And Why Would It Contain Water.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or What Is A Calorimeter And Why Would It Contain Water For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. calorimeter, device for measuring the heat developed during a mechanical, electrical,. What Is A Calorimeter And Why Would It Contain Water.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is A Calorimeter And Why Would It Contain Water For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. To do so, the heat is exchanged with a calibrated object. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can. What Is A Calorimeter And Why Would It Contain Water.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Is A Calorimeter And Why Would It Contain Water To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when an exothermic reaction. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can. What Is A Calorimeter And Why Would It Contain Water.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is A Calorimeter And Why Would It Contain Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the.. What Is A Calorimeter And Why Would It Contain Water.
From www.pinterest.com.au
Pin on Science Friday What Is A Calorimeter And Why Would It Contain Water calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. thermal energy itself cannot be measured easily, but the temperature change. What Is A Calorimeter And Why Would It Contain Water.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is A Calorimeter And Why Would It Contain Water thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in. What Is A Calorimeter And Why Would It Contain Water.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter And Why Would It Contain Water a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. thermal energy itself cannot be measured easily, but the temperature change caused by the flow of thermal energy between objects or substances can be. a calorimeter is a device used to measure the amount of heat. What Is A Calorimeter And Why Would It Contain Water.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter And Why Would It Contain Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. What Is A Calorimeter And Why Would It Contain Water.